EMBO workshop 2021
The tutorials listed here take place virtually on the 11th and 13th of September of 2021 (14:00-18:30) as part of the EMBO workshop for Image processing in cryoEM.
Contents
Connect to Guacamole and run Dynamo
Credentials
Please refer to your personal course material to access your two sets of credentials:
- A set of credentials to enter Guacamole (the platform for connecting via browser). These are common to all participants.
- A set of course credentials to enter the actual machines on which the course will be done: the EMBO21 UserID. These are unique to each participant.
Connect to Guacamole
To connect to Guacamole, first go to the following link in your browser:
https://login.cryst.bbk.ac.uk/guacamole/#/
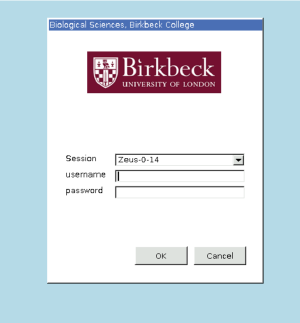
At this stage you should maximize your browser window, otherwise you might have visualization problems when opening Dynamo. You should see the following window. Enter your Guacamole credentials.
Connect to your machine
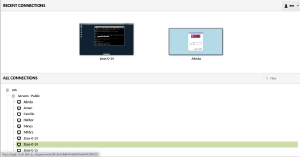
Now you need to select a machine. Please then click on +em and then +Servers - Public and select server Zeus-0-14.
When you select the machine, you will prompted to use your EMBO21 UserID
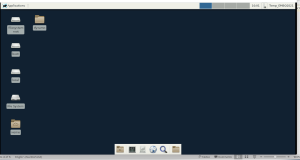
This should access your virtual Linux XFCe desktop, which should look like this in your browser
If you open a terminal (with the terminal icon) you can test by typing
pwd
that you are in the correct path, which should be:
/d/embo2021/u/emboXX
where XX stands for your actual workshop participant number.
Load and run Dynamo
First you load the standalone version of Dynamo:
module load dynamo/v1.1.524
and then it can be run by simply typing:
dynamo
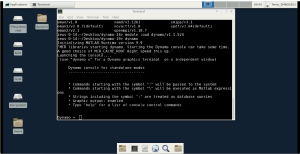
Dynamo specific commands are then typed into this so called Dynamo console. For example, try to type the Dynamo command
dynamo_version
to see the current version that you are using. Note: Copy paste of commands from your local desktop into the dynamo standalone console on the remote Linux XFCe desktop might not always work. We suggest to additionally open the tutorials that you will be working on in a browser inside the remote Linux XFCe desktop itself, in case you need to copy paste long commands.
Data location
The data used in the tutorials is already available for you. Move to the directory of the corresponding practical using the command:
cd prac-5
or
cd prac-6
respectively. Double-check if the data is present by typing
ls
You should see the following files:
Time schedule
The course is divided into different modules, described in the time schedule below. Each module is structured in three stages:
- Demostration: All students are connected to the main room. The goals of the module will be explained by an instructor.
- Individual work: Students go the assigned breakout rooms to work on the tutorials independently. An instructor will be assigned to each breakout room to help with questions.
- Common discussion: In the last 5 minutes of the module, we come back to the general breakout room to wrap up the module.
This time schedule is tentative
Day 1: Tomography
| Time | Module |
| 14:00 | Introduction |
| 14:30 | Tilt series alignment: manually |
| 15:00 | Tilt series alignment: GUI |
| 15:30 | Tilt series alignment: command line |
| 16:00 | Break |
| 16:30 | Models: catalogue system |
| 17:00 | Models: helical symmetry |
| 17:30 | Models: vesicles and surfaces |
| 18:00 | Template matching |
Day 2: Subtomogram averaging
| Time | Module |
| 14:00 | Introduction |
| 14:30 | Getting started: synthetic thermosomes |
| 15:30 | Advanced tutorial: FHV |
| 16:00 | Break |
| 16:30 | FHV: continuation |
| 17:00 | Seed oversampling |
| 18:00 | High resolution pipelines |
| 18:20 | Closing remarks |
Turorials
Alignment of tilt series
We will align the same tilt series tilt series with two different approaches. We prepared the first tilt series from the EMPIAR entry 10164, depicting a set of virus like particles (VLP). Move to the directory that contains this data using the command:
cd prac-5
Double-check by typing
ls
to see all files in the directory. One file should be the following tilt series:
b001ts001.mrc
This is a binned version (for quicker processing) with the pixel size is 2.7 Angstrom (original deposition is 1.35).
Automated alignment of tilt series through the GUI
Walkthrough on GUI based tilt series alignment
Automated alignment of tilt series through the command line
Walkthrough on command line based tilt series alignment
Models
Catalogues
Helical models
Vesicle and surface models
tutorial on membrane modeling with dmslice
Reusing models
Reusing model workflows ( walkthrough)
Template matching
walkthrough for automated identification of proteosomes on a real tomogram through template matching. (~1 hour)
The data is available at:
/d/embo2021/d/dynamo/t20s.mrc
An example of template and its mask are provided as 'average32.em' and 'maskTight32.em'.
Subtomogram averaging
Introduction
Guided presentation:
- Basic Dynamo jargon
- tutorial on basic elements: help, data and metadata formats.
- tutorial on the basic concept in Dynamo alignment: the project.
Working on your own:
Basic walkthrough: creating a catalogue, picking particles, launching a project.
Advanced starters guide
Complete the advanced starters guide (~2 hours) The data can be found in
/d/embo2021/d/dynamo/crop.rec
To use chimera path you need in the tutorial is
TBI
Seed oversampling
Tutorial from densely packed spherical geometry (~1 hour). The data is available at:
/d/embo2021/d/dynamo/v17.rec
Since the final alignment project is run on the standalone version of Dynamo, the 'destination' parameter should be 'standalone' instead of 'matlab_parfor'
High resolution pipelines
VLPs
Exercise to train a realistic case including all processing steps from catalogue creation to final structure using a reduced dataset. Start with the exercise and refer to the complete walkthrough for guidance or the solution.
Interconnectivity tools
Alister: Not a tutorial per se: this is a presentation on a set of tools to interconnect Dynamo, M and other software.
A set of tools have been developed which provide a means to use the geometrical tools and alignment projects from Dynamo with data preprocessed in Warp. These tools mean that particle picking and alignment can be performed in Dynamo prior to multi-particle refinement in M.